Enough oxygen to support 8 billion people for 100,000 years to be exact.
And as Selena is the most suitable celestial body to be colonized, something that has been certified by NASA for 13 years, I expect the discovery to accelerate future projects. In fact, there is already a dilemma about how such a process would affect the human race in the long run, right?
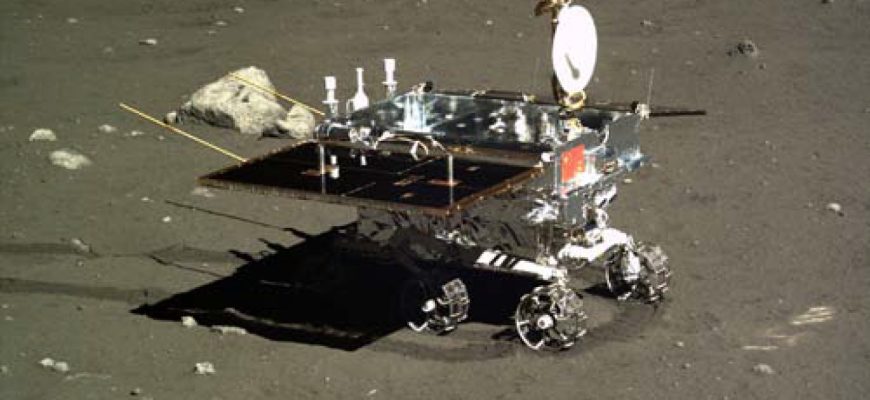
In October, the Australian Space Agency and NASA signed an agreement to send an Australian-made rover to the moon as part of the Artemis program to collect lunar rocks that could eventually provide breathable oxygen to the moon. Although the Moon has an atmosphere, it is very thin and is composed mainly of hydrogen, neon and argon. It is not the kind of gas mixture that could sustain oxygen-dependent mammals, such as humans.
That being said, there is actually a lot of oxygen on the moon. It’s just not in gaseous form, though. Instead, it is trapped inside the regolith – the layer of rock and fine dust that covers the surface of the star. If we could extract oxygen from regolith, it would be enough to sustain human life on the moon, says the recent study.
Oxygen can be found in many of the minerals in the earth around us. And the Moon is made mostly of the same rocks that you will find on Earth (although with a slightly larger amount of material that came from meteors).
Minerals such as silicon, aluminum, and iron and magnesium oxides dominate the Selenium landscape. All of these minerals contain oxygen, but not in a form that our lungs can access. The result is a matrix of minerals that were not present in the original rocks. The soil of the Earth is impregnated with remarkable physical, chemical and biological characteristics. Meanwhile, the materials on the Moon’s surface are practically regulated in its original form, which consists of about 45% oxygen. But that oxygen is closely related to the minerals mentioned above. To break those strong ties, we need to make some effort.
Extraction of oxygen from regolith would also require substantial industrial equipment. We should first turn the solid metal oxide into a liquid form, either by applying heat or combined heat with solvents or electrolytes. We have the technology to do this on Earth, but moving this device to the moon – and generating enough energy to run it – will be a daunting challenge.
Each cubic meter of monthly regolith contains an average of 1.4 tons of minerals, including about 630 kilograms of oxygen. NASA says people need to breathe about 800 grams of oxygen a day to survive. So 630 kg of oxygen would keep a person alive for about two years (or a little over).
Now suppose that the average depth of the moon’s moon is about ten meters and that we can extract all the oxygen from it. This means that the first ten meters of the moon’s surface would provide enough oxygen to support all eight billion people on Earth for about 100,000 years.
Now it remains to be seen who first claims ownership of these reserves, among the states of the world. I know, I exist.